Species Composition - Nested Circles Method
Retired
In use from 2008-01-01 to 2010-12-31
Abstract
Location: Extensive sites of the GLBRC Biodiversity Experiment
Background:
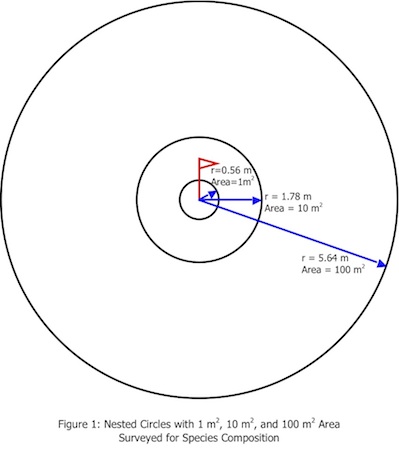
This sampling protocol is an additive radii modification of R. H. Whittaker’s plant diversity sampling method (1). The sample areas are nested circles based at a fixed central point. Species composition is determined at 3 scales (See Figure 1)—
1 m2, radius = 0.56 m
10 m2, radius = 1.78 m
100 m2, radius = 5.64 m
Area of Circle = π r2; π = 3.14
Four non-overlapping stations are sampled in each field site. The location of the stations is established by the GLBRC insect diversity assessment team and marked with a stake and tall flag. This flag is the central anchor point for the nested circle areas.
Protocol
Materials & Equipment:
Meter tape with measurements at 0.56 m, 1.78 m, and 5.64 m highlighted.
Stake & tall flag to mark anchor point
Folding pocket magnifier (Bausch & Lomb)
Clipboards
Field data sheets
Pencils
Wire flag
Method:
1. Place the loop at the zero measurement of the meter tape on the flag pole—this is the fixed central point. Walk away from the central point. Extend the meter tape without slack to 5.64 meters, the radius of the largest area. Place a wire flag at this point to mark the beginning of the survey.
2. Ideally, two people work as a team on the census. One person holds the tape at 5.64 meters from the central point and walks slowly in a circle. A second person walks along the tape moving back and forth from the central point. The circle is walked once, pausing as needed to observe along the length of the tape. Between the two people, the entire 100 m2 area is censused and the smallest area circle where each species first occurs is logged on a field data sheet.
3. All species observed in the circle with a radius of 0.56 meters are logged as present in 1 m2. Any additional species present when the observation is extended to a radius of 1.78 meters are logged as present at 10 m2. Additional species present when the observation is extended to a radius of 5.64 meters are logged as present at 100 m2. Each species present is logged only once at the smallest scale where observed.
4. Because the assessed areas are nested, the complete list of species present at 10 m2 includes the species observed at 1 m2 and 10 m2. The complete list of species present at 100 m2 includes the species observed at 1 m2, 10 m2, and 100 m2.
Notes:
1. Senesced plants that are part of the current year’s biomass and can be identified are included in the census.
2. Newly germinated plants with only cotyledons or fewer than two true leaves are not logged.
3. Plants with white and/or red foliage and no green color have been observed in some corn fields. Plants with no green color are not logged in the census.
4. Only plant species rooted in the census area are logged.
Reference:
(1) Shmida, Avi. 1984. Whittaker’s Plant Diversity Sampling Method. Israel Journal of Botany 33: 41-46.
Plant Identification Keys and Resources:
Gleason, Henry A. and Arthur Cronquist. 1991. Manual of Vascular Plants of Northeastern United States and Adjacent Canada, Second Edition. New York Botanical Garden: Bronx, NY.
Holmgren, Noel H. 1998. Illustrated Companion to Gleason and Cronquist’s Manual: Illustrations of the Vascular Plants of Northeastern United States and Adjacent Canada. New York Botanical Garden: Bronx, NY.
Voss, Edward G. 1972. Michigan Flora, Part I: Gymnosperms and Monocots. Regents of the University of Michigan: Ann Arbor, MI.
Voss, Edward G. 1985. Michigan Flora, Part II: Dicots (Saururaceae–Cornaceae). Regents of the University of Michigan: Ann Arbor, MI.
Voss, Edward G. 1996. Michigan Flora, Part III: Dicots (Pyrolaceae–Compositae). Regents of the University of Michigan: Ann Arbor, MI.
Integrated Taxonomic Information System (ITIS). http://www.itis.gov/
United States Department of Agriculture, Natural Resources Conservation Service PLANTS Database. http://plants.usda.gov/
Date modified: Tuesday, Oct 24 2023
